Cycles of concentration are defined as:
Impurity in the Cooling Tower
Impurity in Feed Water
Example:
| Calcium Cooling Tower | 100 | 200 | 45 |
| Calcium in Feed Water | 20 | 50 | 15 |
| Cycles of Concentration | 5 | 4 | 3 |
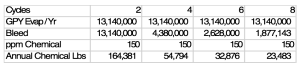
Cycles of concentration are important as the higher the cycles of concentration the lower the bleed will be since bleed as a percentage of feed water = 1/ cycles
Total Water used in a Cooling Tower = Evaporation + Bleed + Drift + Leaks
There are about 1.5 gallons of water evaporated / tn of cooling. If we are running a 1000 Tn system let’s examine the impact of cycles on annual antiscalant usage.