No Nonsense Water Treatment – Evaporation – Cooling Tower
Water changes phase from solid to liquid to vapor. At standard conditions:
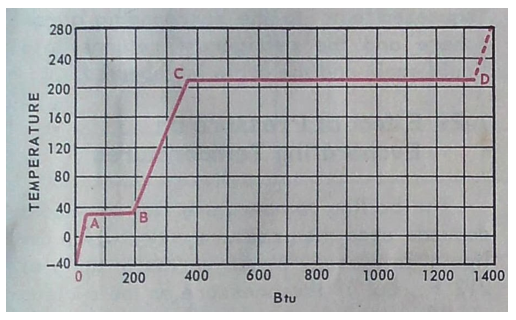
At temperatures less than A ( 32 F ) water is a solid – ice
Heat is added from A – B to melt the ice to water but temperature does not change. The amount of heat needed to do this is 144 BTU / Lb. this is latent ( hidden ) heat as you can’t feel it
More heat is added from B to C increasing the temperature of the water but not changing the phase – water remains a liquid. This is sensible heat as you can feel it.
Heat is added from C to D to change the water to steam but temperature does not change. The amount of heat required to do this is 970 BTU / Lb. this is latent heat.
Once point D is passed water vapor ( steam ) gets hotter and hotter. This is sensible heat.
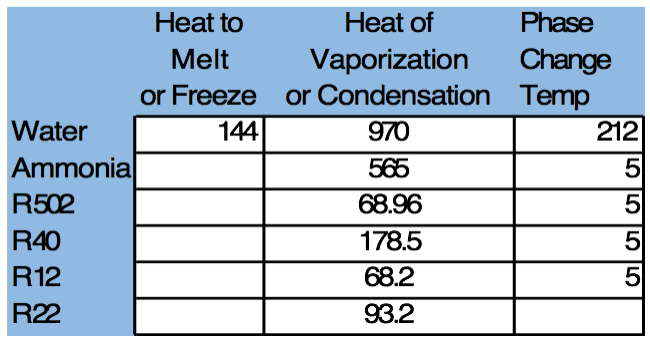
Since 1 Tn of refrigeration is defined as the removal of 12,000 BTU / Hr we can see that a cooling tower must evaporate 12,000 BTU / 970 BTU / Lb. = 12.37 Lbs. of water = 12.37/8.34 gallons / Lb. = 1.48 or about 1.5 gallons of water per hr. to deliver 1 tn. of cooling.
Example
If a cooling tower is running 750 GPM or 43200 GPH and has a 10 degree change in Temperature:
43200 x 8.34 x 10 /12000 = 300.24 Tns of cooling are being provided
